download:300 IF:20
Yue Xinni, Shanxi Yixue Technology Specialized College
Kong Xiangtong (corresponding author)
abstract
background:
With the improvement of the quality of life and the acceleration of the pace of life, cerebrovascular disease has risen to the first cause of death in China. In recent years, the incidence of cerebral infarction (cerebral infarction) is high in China, and its fatality rate and disability rate also show a continuous upward trend, which seriously endangers human health. Among them, the high disability rate not only causes double pressure to the physical and psychological patients, but also makes the economic burden of the family become heavy. The development of modern medical technology has made significant progress in the prevention and treatment of cerebral infarction, but the reduction of disability and mortality is still the focus of frontline clinical doctors. Nerve growth factor (nerve growth factor, NGF) can regulate multiple stages of the growth and development of nerve cells, and its biological characteristics promote injured nerve repair, making it has important clinical significance. At present, many domestic and foreign research institutions have carried out in-depth research on NGF, and its clinical value for cerebral infarction has attracted more and more attention. At present, there are few studies on the relationship between acute cerebral infarction and serum NGF in China.
Objective: In this study, to investigate the relationship between serum NGF and acute cerebral infarction was determined by measuring serum NGF content in patients with acute cerebral infarction.
Methods: The study group included 90 patients with acute cerebral infarction within 24 hours, including 47 males and 43 females aged 43 to 78 years, mean age of 62.19 ± 6.34 years; the control group 20 were outpatient patients, including 11 males and 9 women, aged 44 to 75 years with mean age of 63.57 ± 6.22 years. According to the principle of lesion classification of cerebral infarction, the study group of acute cerebral infarction was divided into small infarction group (maximum diameter of lesion <15mm), medium infarction group (maximum diameter of 15mm lesion <50mm) and group of large infarction (maximum diameter of lesion 50mm). All the subjects of cerebral infarction group, 5ml of venous blood 1d, 7d and 14d, the specimens were stored in-70℃ refrigerator, and the serum NGF level was measured by enzyme-linked immunosorbent (Enzyme-Linked ImmunoSorbent Assay, ELISA) method. The serum NGF content of 20 healthy control subjects were tested in the same way. Measurement data are expressed as mean ± standard deviation (± s), and statistical processing is analyzed by SPS S22.0 statistical software.![]()
Results: 1. The serum NGF content (ng/L) was 16.04 ± 1.33,1d, 7d and 14d, 12.26 ± 1.90 and 9.70 ± 2.73, respectively, and 5.43 ± 1.27. The statistical treatment results showed that the serum NGF content in 1d, 7d and 14d was significantly higher than that of the control group (P <0.01). Meanwhile, the serum NGF content at each time point in the cerebral infarction group (P <0.01).
2. Changes in serum NGF content in patients with different lesion sizes and onset time of acute cerebral infarction, In the group of large infarction, the serum NGF content (ng/L) at 1d, 7d and 14d were 18.31 ± 0.46,14.94 ± 0.62 and 13.71 ± 0.63, respectively; The serum NGF content (ng/L) at 1d, 7d and 14d in the middle infarction group was 16.20 ± 0.42,13.08 ± 0.73, and 10.76 ± 1.11, respectively; Serum NGF content (ng/L) in the cases at 1d, 7d and 14d were 14.96 ± 0.39,10.54 ± 0.81 and 7.32 ± 1.06, respectively. The statistical treatment results showed that the serum NGF content was significantly different at different time points (P <0.01).
conclusion:
1. The serum NGF content of patients with acute cerebral infarction has a dynamic trend of rising first and then decreasing.
2. Serum NGF content was positively correlated with the size of cerebral infarction in patients with acute cerebral infarction.
3. The detection of serum NGF level in the acute phase of cerebral infarction patients may have an important reference value for assessing the severity of the disease.
Key words: nerve growth factor; acute cerebral infarction; enzyme-linked immunosorbent method
第一章 foreword
Cerebral infarction refers to the disease of cerebral blood circulation disorders caused by various reasons, resulting in ischemia and hypoxia of the corresponding brain tissue, and finally producing tissue necrosis and softening forming infarction. It is the most common clinical cerebrovascular disease, with the characteristics of high fatality rate, high disability rate and high recurrence rate. In recent years, due to the rapid development of medical imaging, the popularization of CT and MRI for patients with cerebral infarction can be detected in time, these was found in the early patients makes the incidence of cerebral infarction, patients not only to bear heavy medical economic burden, suffer the psychological blow, so do the work of cerebral infarction prevention and treatment is particularly important, to further explore and seek more optimized prevention and control measures are urgent.
Neurotrophic factor (neurotrophin, NT) is a class of protein molecules necessary for the growth and survival of nerve cells. NGF belongs to the neurotrophic factor family, and is the first neurotrophic factor discovered in humans. NGF can regulate the growth and development of nerve cells, maintain the survival of mature nerve cells and repair the damaged nerves. These characteristics make many domestic and foreign experts attach great importance to the research of NGF in the field of cerebral infarction. As the clinical value of NGF is constantly explored, NGF is expected to become a new entry point for the diagnosis and treatment of cerebral infarction. Improving the expression of endogenous NGF and the use of exogenous NGF may become a new path for the diagnosis and treatment of cerebral infarction, reducing the disability rate and mortality.
At present, many research institutions have carried out research and development on NGF, most of which focuses on exogenous NGF. Although the study of endogenous NGF has attracted the attention of many experts and scholars, there are few clinical studies related to cerebral infarction. In this study, we examined the relationship between serum NGF content and acute cerebral infarction in the acute phase of cerebral infarction.
第二章 Review on the relationship between acute phase cerebral infarction and serum NGF
二.1 The biological basis of the NGF
二.1.1 The basic structure and distribution of NGF
NGF is the first neurotrophic factor [1] discovered by Italian scientist Rita Levi-Montalcini in mouse sarcoma cells in 1953. It is a soluble basic protein with sedimentation coefficient of 7S, and the gene code is located in chromosome 1 (1P21-P22.1). The genetic information is transcribed and translated to form three subunits of α, β, and γ. Under the force of non-covalent bonds, it becomes a poly complex in the form of α2β2γ2, with high relative molecular mass of 14010 ³ kDa. Among them, the β subunit assumes the vast majority of the active functions of NGF. This subunit has 236 amino acids, which is a double-stranded polypeptide maintained by non-covalent bonds, and the regions related to biological functions are highly conserved in evolution. There are six special cysteine residues in NGF, which form three pairs of disulfide bonds and play a crucial role in the biological activity of NGF. Once the disulfide bond is hydrolysed, NGF will also lose its bioactive [2-3]. NGF also has high homology, especially in molecular biological activity without obvious species specificity, purified from the male mouse submandibular gland of mNGF and human NGF homology has reached more than ninety percent and target cell biological effect difference, this characteristic makes mNGF is widely used in scientific research and clinical treatment of [4].
In the nervous system, NGF is mainly produced in the projection area of the central cholinergic nucleus, so the cholinergic nerve cells is the main distribution area. The cholinergic neurons in the cerebral cortex and hippocampus are very rich, so NGF in the brain is highest distributed in these two parts. The distribution of NGF in other parts of the brain is successively from high to low: olfactory bulb, basal forebrain, cerebellum and striatum. The unbalanced distribution in the brain also reflects the difference in the content of cholinergic nerve cells in different brain tissues [5]. In addition, NGF is widely distributed in other parts of the body: [6] such as salivary glands, prostate, and placental tissues.
二.1.2 The receptor for NGF
There are two main receptors for NGF, namely tyrosine kinase A (tyrosine kinase receptor A, TrkA) and neurotrophin p75 (neurotrophin receptor p75) [7]. The former has strong affinity and can specifically bind to NGF, trigger the activation of related proteases, initiate a series of pathways of signaling, and finally enable the biological effects of NGF to be expressed. Therefore, TrkA is a functional receptor of NGF, with [8] throughout the cell membrane of NGF effector cells. The p75 is a class of cysteine-rich transmembrane glycoproteins with a very wide distribution, diffuse throughout the central nervous system and also in the nervous system. With a much lower affinity than TrkA, Also are unable to produce direct biological effects after binding to NGF, But it has many irreplaceable functions [9]: (1) can accurately identify the NGF family, And combined with the family members; (2) can regulate the activity of tyrosine kinase, Enhance the binding ability of TrkA to NGF, Make the full expression of the biological effects of NGF; (3) Reverse delivery of NGF, Especially in the repair of damaged peripheral nerves; (4) aggravate the rate of sphingomyelin hydrolysis, Guide cell apoptosis; (5) excitation guanylate binding protein coupling mechanism, Ensure the stable transmission of biological signals.
二.1.3 Signaling pathways of NGF
NGF has two receptors, TrkA and p75, and because they differ significantly in various aspects, they each mediate different signaling pathways, [7].(1) TrkA generally mediates positive signals, such as promoting the survival, growth, and differentiation of nerve cells. That TrkA generates dimers on the membrane after binding to NGF, Initiautophosphorylation of multiple tyrosine residues of Trk A, In turn to activate downstream signaling proteins, In the order in which the tyrosine residues are activated, The TrkA-mediated signaling pathway depends on the coordinated operation of these three pathways: ① mitogen-activated protein kinase (mitogen-activated protein kinase, MAPK) pathway: the residue Y490 of TrkA is first phosphorylated, Further activation of the MAPK kinase, To open up the MAPK pathway, MAPK switched on the B lymphocyoma-2 gene (B-cell lymphoma-2, The expression of Bcl-2), Play the biological effect of inhibiting apoptosis.② Phosphoesterase C- γ (phospholipase C- γ, PLC- γ) pathway: the phosphorylation of TrkA residue, after a series of biochemical reactions eventually activate extracellular regulatory protein kinase (extracellular regulated protein kinases, ERK), producing the biological effects of enhancing the vitality and regeneration of nerve cells.③ Phosphatidylinositol-3-hydroxykinase (phosphatidylinositol 3-hydroxy kinase, PI3K) / protein kinase B (protein kinase B, PKB) pathway: TrkA phosphorylated residue Y751 is linked to PI3K, PI3K can activate PKB and enhance Bcl-2 transcription, PKB and Bcl-2 jointly enhance the survival of nerve cells.
(2) p75 mainly conducts negative signals that induce apoptosis, but also can mediate a small number of positive signals that promote the survival of nerve cells. The pathways to conduct signaling after p75 binding to NGF include: ① nuclear transcription factor- κ B (nuclear factor kappa-light-chain-enhancer of activated B cells, NF- κ B) pathway: In the study of [10] in the rat model of cerebral ischemia and reperfusion, [10] found that the gene coding products regulated by NF- κ B have antioxidant effects and enhanced cellular defense. Activation of NF- κ B can exert its effect against apoptosis while enhancing the activity of TrkA.② c-Jun amino-terminal kinase (c-Jun N-terminal kinase, JNK) -p53-BAX pathway: This pathway can mainly improve the speed of apoptosis, JNK, p53 and BAX are initiated in turn, in which the content of p53 is directly related to apoptosis [11].③ Ceramide pathway: The biological effects mediated by this pathway have dual effects [12], which is related to the concentration of protein kinase C (protein kinase C, PKC) associated with ceramide. Low concentration of ceramide can activate PKC, promoting the growth and development of nerve cells, while high concentration of ceramide reduces PKC activity, leading to the rapid death of nerve cells.
As mentioned above, TrkA mediates positive signals and p75 mainly transmits negative signals, but in the vast majority of cellular background and environment, TrkA can effectively inhibit p75 induced negative signal [13], finally enabling the total biological effect of NGF to promote the growth and development of nerve cells and enhance the repair of diseased nerves.
二.2 The biological effects of NGF
(1) In the early stage of nervous system development, NGF can regulate the survival of nerve cells and promote the differentiation and development of nerve cells by promoting the growth and differentiation of neural stem cells, regulating the synthesis of neurotransmitters and inhibiting the apoptosis of nerve cells [14,15].
(2) NGF can also enhance the viability of a variety of mature nerve cells and promote the connection between the axons of mature cells and other cells, so it has the biological effect [16] to maintain the mature stage of the nervous system.
(3) When the nervous system encounters damage, the expression of NGF is rapidly increased, which protects the damaged nerve cells by regulating the environment around the cells. NGF promotes axonal regeneration of [17], which can form functional connections with target cells, and enhance the myelin repair force [18], finally making the injured nerve cells complete the repair and achieve the purpose of improving the symptoms of neurological deficiency. The biological effects of NGF in protecting and repairing the diseased nervous system are effective for both the central nervous system and the peripheral nerves.
(4) In addition, through the in-depth study of NGF in recent years, it is found that it also has certain effects on the non-nervous system, such as regulating the immune system function, promoting fracture healing, affecting the inflammatory reaction and other [19].
二.3 Pharmacological progression of NGF
NGF has promising clinical applications due to its unique biological effects and may become a potential therapeutic agent for many diseases, such as optic nerve injury, cerebral infarction, Alzheimer's disease, craniocerebral trauma, spinal cord injury and peripheral neuropathy. Male mice submandibular gland extraction of mNGF has the characteristics of safe, effective and low side effects, is the most commonly used can meet the scientific research and clinical application of N GF, injection with mouse nerve growth factor (after complex) has been listed in China, pharmacokinetics show the rat after muscle peak time for 4 hours, 24 hours absorption rate is higher than 80%, mainly from the kidney, few excreted with feces. Murine nerve growth factor for injection has the efficacy of treating toxic peripheral neuropathy in n-hexane and promoting the recovery of nerve injury, which brings good news to many patients.
Most of the clinically applied NGF are administered by intramuscular injection, and the vast majority of them are used for the treatment of peripheral nerve lesions. NGF has a relatively large molecular mass, and it is difficult to cross the blood-brain barrier (BBB) in the natural state, which has little effect on the treatment of internal diseases in the brain. BBB is a special barrier between blood and brain tissue. It is composed of vascular endothelium, basement membrane and astrocyte end feet, which can selectively restrict part of the material in and out of brain tissue, and has certain protection for the brain. In recent years, many studies have found that BBB can increase permeability under some conditions: Li Bing et al. found that BBB can open to a certain extent after craniocerebral injury, the permeability of opening is positively correlated with the degree of injury, and NGF can enter the brain tissue with the opening of BBB [20]. Zhou Wenli and Yan Chaoying found through the experiments of rats that brain hypoxia could increase the permeability of BBB, which was beneficial to exogenous NGF in the brain tissue [21]. Liu Zengxu et al. have studied the tight junctions between vascular endothelial cells in depth, and found that if the structure and function of tight junctions are changed by trauma, ischemia, hypoxia, infection, immunity and physical and physiochemical factors, the increase of BBB permeability will cause [22] to varying degrees. Based on the above studies, it can be speculated that the mechanism by which NGF can pass through BBB during cerebral ischemia may be as follows: (1) the structure of BBB is destroyed: vascular endothelium and astrocyte end feet are the two major constituent structures of BBB. The tight junctions between vascular endothelial cells are underlying the selectivity of BBB, and when cerebral ischemia occurs, the tight junctions between vascular endothelial cells become wide and loose, resulting in increased BBB permeability [22]. Astrocytes play a key role in maintaining the function of BBB. Cerebral ischemia will enlarge the foot mutation of astrocytes and cause increased blood-brain barrier permeability [23].(2) Opening of special transport pathway: cerebral ischemia can initiate unsaturated transmembrane diffusion and saturated transport of specific transport proteins, [24], so that macromolecules such as NGF can easily pass through BBB. After the occurrence of cerebral ischemia, NGF enters the blood from the brain tissue through BBB with increased permeability, which enables NGF to be detected in the peripheral blood. Therefore, ischemic cerebrovascular disease is closely related to the serum NGF content.
Although under some conditions, BBB is open due to increased permeability, and NGF can freely enter and exit BBB, it is difficult to maintain an ideal concentration in brain tissue and enable it to exert stable biological effects. For the dilemma of NGF in its clinical application, At present, many domestic and foreign experts have conducted in-depth research on NGF, Proposed new drug delivery routes, such as recombinant cell injection method, catheter extracerebral injection method, special eye drop method, neural pathway delivery method, polymer implantation method, chemical connection method, biotin-anti-biotin protein technology [25-27]; Ding Y et al [28] found through a transient ischemia model in the middle cerebral artery, Exercise can improve the intracranial NGF expression; Huang's team sealed NGF in nanoflexible liposomes, By experimentally demonstrated that NGF nanoloaded flexible liposomes can effectively promote their breakthrough BBB [29], The above studies are expected to broaden the clinical application field of NGF.
二.4 Changes in the content of NGF in the ischemic brain tissue
In the normal physiological state, NGF is low in brain tissue, but it is significant for nerve cell growth and development, maturation and repair after injury. Liu peng group [30] found that (Sprague-Dawley, SD) rat focal cerebral ischemia, cortical ischemic penumbra NGF positive nerve cell number is higher than the sham group, suggesting that brain tissue ischemia can upregulate ischemic penumbra NGF expression level, and speculated that the brain tissue in the case of ischemia its endogenous protective mechanism of open and NGF expression in the brain. Lindvall O And other foreign experts [31] found that the expression of NGF mRNA was enhanced 2 hours after cerebral ischemia, and the degree of expression depends on the time and degree of brain ischemia. In China, Zhang Hui et al. [32] used the line plug method to produce the middle cerebral artery occlusion (middle cerebral artery occlusion, MCAO) model in SD rats. After 24h after focal cerebral ischemia, the expression of NGF in nerve cells and glial cells in the ischemic marginal area, among which the expression of NGF in simple cerebral ischemia was more obvious than that in patients with cerebral ischemia combined with other diseases. Wei Menglin, Zou Yu'an and other [33] also obtained similar NGF expression results through related experiments. They found that the expression of NGF in the infarct area of brain tissue in experimental rats increased at the beginning of the disease, and reached the peak in about 22 hours, and then gradually decreased. Duan Shurong, Wang Haitao and others [34] dissected the brains of many patients with cerebral infarction, NGF expression in the subependymal area of the lateral ventrerebiticle and the dentate gyrus of the hippocampus at different time points, Found that the expression of NGF began to increase at 4.5 to 10 hours after ischemia, Peak at 24 to 70 hours after ischemia, 3 to 4 days, More positive cells were still expressed from 5 to 6 days of ischemia, Suggesting that NGF may be involved in the endogenous neuroprotective effects of ischemic brain injury, It was also found that the transforming growth factor- β (transforming growth factor- β, TGF- β), vascular endothelial growth factor (vascular endothelial growth factor, VE GF) overlap with NGF in the time of expression, The protective effects of the three on the damaged nerves after cerebral infarction may be synergistic.
Through the above studies of experts and scholars at home and abroad, it can be found that the release of NGF can be improved after ischemia, and the content of NGF in ischemic brain tissue can increase. And the expression of NGF has a certain regularity.
二.5 look into the distance
NGF is a large molecular protein that promotes the growth, development and repair of nerve cells, and its unique biological effects make it very effective in the treatment of neuropathy, especially in the treatment of peripheral neuropathy. In recent years, on the road of exploring the treatment of central neuropathy, several animal experiments and clinical treatment cases [35-37] show that NGF has great potential in the treatment of diseases such as cerebral infarction. As the research focus of NGF shifts from peripheral nerve to central nerve, the relationship between NGF and cerebral infarction is increasingly concerned: the application of exogenous NGF can improve cognition, language, movement and reduce the disability rate; the content of serum NGF in patients with cerebral infarction may have important reference value for assessing the severity of the condition.
第三章 experimental research
三.1 Data and methods
三.1.1 clinical data
Conditions for inclusion of patients in the cerebral infarction group: patients with acute phase of cerebral infarction from October 1,2015 to December 30,2016, and met the diagnostic criteria [38] of Key Points of Diagnosis of Various Cerebrovascular Diseases formulated by the 4th National Cerebrovascular Academic Conference of the Chinese Medical Association in 1995. Inclusion criteria: (1) admission fashion within 24 hours of onset; (2) MRI examination of the head, clearly showing acute brain infarction; (3) localization signs of significant neurological deficit. Exclusion criteria: (1) patients with critical diseases with other systems, such as severe heart and kidney diseases; (2) patients with dangerous infectious diseases; (3) patients with metabolic disorders or vigorous metabolic diseases; (4) patients with unbalanced immune regulation and abnormal immune function; (5) patients with a history of tumor or organ transplantation; (6) patients with surgery or trauma in the past 4 weeks; (7) patients with severe malnutrition.
According to the above criteria, 90 patients were included, including 47 males and 43 females. They ranged from 43 to 78 years, with a mean age of 62.19 ± 6.34 years. According to Guo Yupu [39] lesion size classification method, the above patients were divided into three groups: small infarction group (maximum diameter of lesion <15mm), middle infarction group (maximum diameter of 15mm lesion <50mm) and group of large infarction group (maximum diameter of 50mm).
After previous medical history inquiry, detailed physical examination and multiple auxiliary examinations to exclude organic diseases, a total of 20 control group in the same period, including 11 men, 9 women, aged 44~75 years, the average age was 63.57 ± 6.22 years. No significant difference in age and sex between the cerebral infarction group and the control group.
三.1.2 specimen collection
In the cerebral infarction group, 5ml of fasting venous blood was collected in the morning on the 1d, 7d and 14d of the disease. The blood samples were centrifuged for 15 minutes. The upper serum was stored in a Eppendorf tube in a-70℃ refrigerator. Fasting venous blood was collected once in the control group in the morning, and the process of collection and treatment was no different from that of the cerebral infarction group.
三.1.3 empirical method
After checking that each sample was free of bubbles, turbid precipitation and repeated freeze and thawing, the serum NGF content was determined according to the operation procedures of the ELISA kit instructions. The linear regression equation of the standard curve is calculated based on the concentration of the standard and the absorbance (A) value of the contrast, and then the corresponding sample concentration is calculated on the regression equation based on the A value of the sample.
三.1.4 Statistical method
The raw data obtained in the experiment was entered into SPS S22.0 software for statistical analysis. The comparison between the two samples was analyzed by t-test, and the comparison between multiple groups was analyzed by variance treatment, and the mean ± standard deviation (± s) was used to represent the obtained measurement data. P <0.05 indicates statistical significance.![]()
三.2 bear fruit
三.2.1 Dynamic changes of serum NGF content at 1d, 7d and 14d in the cerebral infarction group
The contents (ng / L) of serum NGF at 1d, 7d and 14d were 16.04 ± 1.33,12.26 ± 1.90 and 9.70 ± 2.73, and 5.43 ± 1.27 in the control group. After statistical treatment, it was found that the content of serum NGF on 1d, 7d and 14d of cerebral infarction was significantly higher than that of the control group (P <0.01), and there were significant differences between the serum NGF content in 1d, 7d and 14d of cerebral infarction (P <0.01), as shown in Table 3.1 and Figure 3.1.
Table 3.1 Dynamic changes of serum NGF content in patients with acute cerebral infarction period (± s, ng / L)![]()
Group, nNGF |
Control group was 20 5.43 ± 1.27 1d 90 16.04 ± 1.33* The infarct group was 7d 90 12.26 ± 1.90*& 14d 90 9.70 ± 2.73*# |
Note: * indicates P <0.01 for comparison between experimental group and control group, & means P <0.01 for onset 7d vs. onset 1d, and # means P <0.01 for onset 14d versus onset 7d.
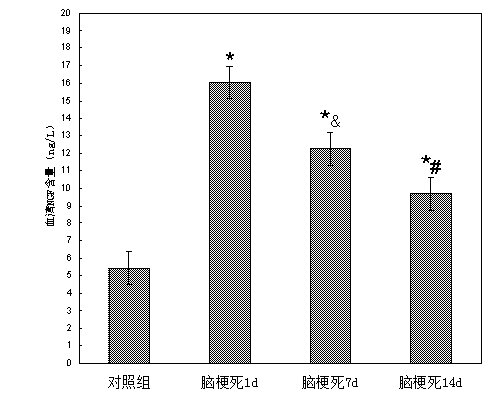
Note: * indicates P <0.01 for comparison between experimental group and control group, & means P <0.01 for onset 7d vs. onset 1d, and # means P <0.01 for onset 14d versus onset 7d.
Figure 3.1 Comparison of dynamic changes of serum NGF content in patients with acute cerebral infarction at different times of onset
三.2.2 Dynamic changes of serum NGF content in patients with different lesion sizes and different time of onset in acute cerebral infarction
In the group of large infarction, the serum NGF content (ng/L) at 1d, 7d and 14d were 18.31 ± 0.46,14.94 ± 0.62 and 13.71 ± 0.63, respectively; The serum NGF content (ng/L) of 1d, 7d and 14d in the middle infarction group was 16.20 ± 0.42,13.08 ± 0.73 and 10.76 ± 1.11, respectively; Serum NGF content (ng/L) in the cases at 1d, 7d and 14d were 14.96 ± 0.39,10.54 ± 0.81 and 7.32 ± 1.06, respectively. After statistical treatment, the serum NGF content was significantly different in all three patients at different time points (P <0.01). See Table 3.2 and Figure 3.2 for details.
Table 3.2 Dynamic changes of serum NGF content in patients with different lesion size and different time of onset in acute cerebral infarction (± s, ng / L)![]()
Group n onset 1d onset 7d onset 14d |
Large infarct group 18 18.31 ± 0.46△14.94±0.62△13.71±0.63△ Middle infarct group 30 16.20 ± 0.42▲13.08±0.73▲10.76±1.11▲ In the small infarct group, 42 14.96 ± 0.39 10.54 ± 0.81 7.32 ± 1.06 |
Note: ▲ indicates P <0.01 for small infarct; △ indicates P <0.01 for large infarct.
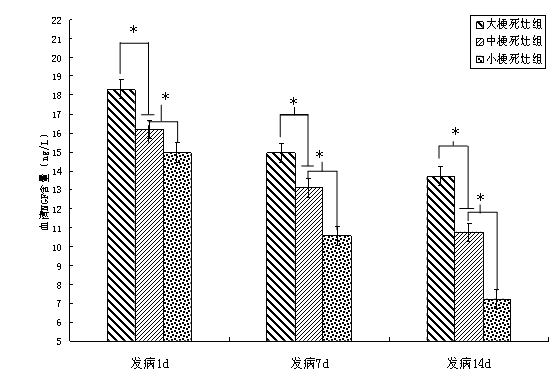
pour:*P <0.01 between the previous group.
Figure 3.2 Comparison of the dynamic changes of serum NGF content at different times of onset and different infarction sizes in patients with acute cerebral infarction
第四章 discuss
NGF is one of the most important nerve cell growth regulators in the nerve growth factor family, which is mainly distributed in the nervous system, especially in the brain. After binding to the corresponding receptor, NGF functions through different signal transduction pathways. In normal physiological state, NGF can promote the growth and differentiation of nerve cells and maintain the mature [40] of nerve cells. When the body is sick, NGF can promote the repair of damaged cells and inhibit the apoptotic [41]. Therefore, NGF has great prospects for clinical application.
The biological characteristics of NGF are mainly manifested in the following aspects: in the early stage of the nervous system, NGF has started to promote the growth, development and differentiation of nerve cells; NGF can improve the viability of mature nerve cells and maintain the mature state of the nervous system; when the nervous system is damaged, highly expressed NGF can stabilize the environment inside and outside the cells and promote the repair of damaged nerve cells; in addition, NGF also has a positive effect on the non-nervous system.
After cerebral tissue ischemia, a small amount of blood seeps around the blocked blood vessels, and the local ischemic brain tissue appears edema. The brain is weak in its tolerance to ischemia and hypoxia, which can cause cerebral infarction [42] in a short time. Cerebral infarction acute phase focus is by a large number of nerve cell death of central necrosis area and peripheral residual collateral compensatory circulation of ischemic penumbra, as much as possible to reduce nerve cell necrosis, save ischemic penumbra is the cause of ischemic brain tissue upregulate NGF expression, NGF can through the following mechanism to protect and repair ischemic brain tissue [43-45]: ① maintain intracellular Ca 2 + concentration stability: brain ischemia can lead to extracellular Ca 2 + influx, make it in the intracellular Ca 2 + overload. NGF maintains the stability of intracellular Ca 2 + concentration through calmodulin, calcium channel and the calcium emission system and protects damaged nerve cells; ② improves free radical clearance: Free radicals are the product of various biochemical reactions in the body, with strong oxidation, and the vast majority of free radicals can attack the human body through various ways. In healthy people, free radicals are in balance, and brain tissue ischemia will break the original order and cause the sharp increase of free radicals, leading to the damage of ischemic brain tissue. NGF can enhance the activity of many free radicals, improve the clearance of free radicals, and reduce the harm of damaged cells; ③ reduces glutamate concentration: glutamate as an excitatory neurotransmitter in the central nervous system, and can participate in many chemical reactions. When brain tissue ischemia, intracellular glutamate concentration often exceeds the standard, causing necrosis of nerve cells. NGF can stabilize the concentration of glutamate through various ways and further slow the concentration surge of many aggressive ions; the negative role of ④ inhibition of carbon monoxide (NO) by ④: NO can play a positive role in enhancing the blood circulation in the ischemic penumbra area in the early stage of cerebral ischemia, but has a negative role in the late stage of aggravating the damage of the central necrotic area. NGF can reduce the enzyme activity of NO generation and reduce some negative effects; ⑤ slows down the apoptosis rate: the environment of cerebral ischemia and hypoxia makes a large number of cell apoptosis factors activated, NGF controls the transcription and translation of Bcl-2 and other proteins, reducing the nerve cell apoptosis rate; ⑥ Other mechanisms: NGF can improve the metabolic rate of damaged nerve cells and increase the synthesis rate of macromolecular material [46]. NGF can induce vascular regenerative [47], promote myelin formation of [48], inhibit the expression of transient receptor potential channel 7 (transient receptor potential melastatin 7, TRPM 7) in hippocampal neurons, and delay cell death [49]. NGF can also stimulate the proliferation of neural stem cells and differentiate into neural cell [50], so that it can partially replace the damaged nerve cells to improve the functional defect of the nervous system.
In Table 3.1 and Table 3.1 and Figure 3.1, the serum NGF content of 1d, 7d and 14 d was higher than that of the healthy control group. The reasons for this result may be as follows: (1) when cerebral infarction occurs, the structure of BBB is destroyed, and the permeability is greatly increased, so that NGF in the brain can enter the blood through BBB, leading to the increase of NGF content in serum.(2) In the peripheral area of ischemic brain tissue, specific nerve cells, some astrocytes, activated microglia cells and macrophages can strongly upregulate the expression of NGF in [51].(3) After the occurrence of cerebral infarction, the activation of SHH (sonic hedgehog) signaling pathway increases the expression of NGF by [52].(4) After the occurrence of cerebral infarction, the expression of a series of special genes and protein synthesis will be induced, such as early genes (immediate early genes, IEGs), interleukin-1 (Interleukin-1, IL-1) and tumor necrosis factor (tumor necrosis factor, TNF). The expression and protein synthesis of such genes can initiate or induce the expression of NGF [53,54]. Cerebral infarction group onset 1d serum NGF content is the highest, with the onset of time, cerebral infarction group serum NGF content decreased in turn, at the onset of 14d serum NGF content, the cause of this result may be as follows: (1) NGF effect on diseased nerve cells play a biological effect, with the progress of the disease, its gradually consumed, so the content in the blood.(2) After the treatment of patients with cerebral infarction, the injured BBB is gradually repaired, and its barrier effect is gradually restored. The NGF in the brain is difficult to enter the blood through the BBB, and its serum concentration is naturally reduced.(3) With the correction of cerebral ischemia, the inducement or pathway promoting the expression of NGF was gradually weakened, and the resulting NGF content was reduced, and the serum NGF content decreased. Comprehensive above experimental results we know that the acute cerebral infarction serum NGF content increased significantly, NGF expression after brain tissue ischemia, may begin to play a biological effect in the early stage of cerebral ischemia, over time its content gradually reduced but still higher than healthy controls, this shows that in the late ischemia repair process, NGF still plays an important role.
Table 3.2, Figure 3.2 and Figure 3.3 showed that the serum NGF content was correlated with the size of the infarct in the acute group of cerebral infarction, and the larger the infarction, the higher the serum NGF content, that is, the serum NGF content was positively correlated with the size of the cerebral infarction lesion. The reasons may be as follows: (1) the heavier the condition of cerebral infarction, the larger the area of the corresponding infarct, in order to protect and repair the damaged brain tissue, the stronger the body stress response, and the amount of induced NGF expression will increase.(2) The larger the cerebral infarction focus is, the heavier the BBB damage will be. With the decline of BBB barrier effect, the NGF produced by brain tissue can enter the blood more, causing the increase of serum NGF content. Serum NGF level in the acute phase of cerebral infarction may be important for assessing the severity of cerebral infarction.
Foreign scholars Takeda A, Hsu CY and others found that through animal experiments [55,56], the elevated expression of NGF could be observed in either transient or prolonged brain ischemia. Through the clinical study of 30 patients with acute cerebrovascular disease, domestic physician Huang Jian et al found that the changes of NGF in hemorrhagic and ischemic stroke patients were the same: the content of NGF in blood increased significantly 3d after onset, reached the peak at 1 week, and basically returned to normal after 1 month; the content of NGF in blood in the early onset and increased significantly in patients with moderate onset. The above research results are similar to our research results.
Maintaining the ischemic penumbra tissue around the cerebral infarction area in the acute stage of cerebral infarction is the top priority of treatment. Patients with cerebral infarction in the acute phase of the BBB permeability increase, can make NGF free in and out of the BBB, in the acute phase timely supplement exogenous NGF can protect ischemic tissue nerve cells have not yet necrosis, reduce the damage of cerebral ischemia, improve nerve defect symptoms, improve the prognosis recovery, and effectively improve patient quality of life, this has been confirmed in the clinical research of many researchers and medical workers. It is believed that in the near future, NGF can play an important role in the nervous system or more fields.
第五章 conclusion
1. The serum NGF content of patients with acute cerebral infarction has a dynamic trend of rising first and then decreasing.
2. Serum NGF content was positively correlated with the size of cerebral infarction in patients with acute cerebral infarction.
3. The detection of serum NGF level in the acute phase of cerebral infarction patients may have an important reference value for assessing the severity of the disease.
reference documentation
[1]Rita Levi-Montalcini.The Nerve Growth Factor:thirty-five years later[J].The EMBO Journal,1987,6(5):1145-1154.
[2] Xu Li. Progress in nerve growth factor research [J]. Chinese Journal of Biological Products, 2014,27 (1): 131-134.
[3] Kong Xiliang, Liu Hongzhen. Recent research status of nerve growth factor [J]. Clinical Rehabilitation in China, 2004,8 (10): 1920-1921.
[4]Cohen S.Purification of a nerve-growth promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum[J].Proc Natl Acad Sci USA,1960,46(3):302-311.
[5]Shelton DL,Reichardt LF.Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system:level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons[J].Proceedings of the National Academy of Sciences of the United States of America,1986,83(8):2714-2718.
[6] Huang Junhong, Tan Aoyong, Tan Zhanguo. Progress in nerve growth factor in the repair of central nervous function [J]. Chinese Journal of Practical Neurological Disorders, 2014,17 (19): 126-127.
[7] Peng Fengling, Mo Zhongcheng, Zheng Xiang, etc. Progress in signaling pathways associated to nerve growth factors and their receptors [J]. Advances in Modern biomedicine, 2015,31 (15): 6190-6193.
[8] Yin Fangming. P75 and TrKA: two NGF acceptors [J]. The International Journal of Neurology and Neurosurgery, 1997 (4): 205-208.
[9] Zhang Linkun, Zhang Yincheng. Advances in nerve growth factor and gangliosides [J]. Chinese Journal of Practical Neurological Disorders, 2010,13 (10): 89-93.
[10] Xing Xuesong, Lv Wei Li, Zhang Guobin. Modulation of apoptosis and nuclear factor- κ B expression in calcitonin gene-related peptides and nerve growth factor [J]. Journal of Anatomy, 2007,30 (4): 320-322,350.
[11]QG He,L Yu,W Xu,J Duan,J Guo,et al.Abstract 2473:Ubiquitin Ligase Huwe1 Modulates Prongf/p75ntr In Monkey Cerebral Ischemia-reperfusion Injury[J].Stroke,2012(2):A2473.
[12]P Nair,S Tammariello,S Estus.Ceramide selectively inhibits apoptosis-associated events in NGF-deprived sympathetic neurons[J].Cell Death & Differentiation,2000,7(2):207-214.
[13]Lloyd A Greene,David R Kaplan.Early events in neurotrophin signalling via Trk and p75 receptors[J].Current Opinion in Neurobiology,1995,5(5):579-587.
[14]Chiaretti A,Genovese O,Riccardi R,et al.Intraventricular nerve growth factor infusion:a possible treatment for neurological deficits following hypoxic-ischemic brain injury in infants[J].Neurol Res,2005,27(7):741-746.
[15]Schumacher AM,Velentza AV,Watterson DM,et al.DAPK catalytic activity in the hippocampus increases during the recovery phase in an animal model of brain hypoxic-ischemic injury[J].Biochim Biophys Acta,2002,1600(1-2):128-137.
[16]Hellweg R,Fischer W,Liock C,et a1.Nerve growth factor levels and choline acetvltransferase activity in the brain of aged rats with spatial memory impaiment[J].Brain Res,1990,537(12):123-130.
[17]Bonilla IE,Tanabe K,Sm S.Small proline-rich repeat 1A is expressed by axotomized neurons and promotes axonal outgrowth[J].Neurosci,2002,22:1303-1315.
[18]Hudson Tw,Evalls G,Ce S.Engineering strategies for peripheral nerve repair[J].Orthop Clin,2000,1l(3):485-497.
[19] The Chinese expert consensus collaboration group on the clinical application of nerve growth factor. Expert consensus on the clinical application of nerve growth factor (EnF) [J]. Chinese Journal of Neuromedicine, 2012,11 (4): 416-420.
[20] Li Bing, Lu Xiaojie, Chen Jian, Luo Zhong Zhong. Nerve growth factor BBB permeability after rat brain injury [J]. Chinese Journal of Neurosurgical Disease Research, 2008,7 (3): 216-219.
[21] Zhou Wenli, Yan Chaoying, Zhang Jiantao, Zhao Jingwei. Penetrability of exogenous nerve growth factor to the blood – brain barrier and its brain tissue distribution in hypoxic brain-injured neonatal rats [J]. Chinese Journal of Biological Products, 2010,23 (3): 261-263.
[22] Liu Zengxu, Wang Hanghui, Wang Xiangdong, Liu Deming. Progress in the molecular composition and signal regulation of the tight junctions of the blood-brain barrier [J]. Journal of Jiangxi Medical College, 2006,46 (5): 173-175.
[23]Kondo T,Kinouchi H,Kawase M,et al.Astroglial cells inhibit the increasing permeability of brain endothelial cell monolayer following hypoxia reoxygenation[J].Neurosci Lett,1996,208(2):101-104.
[24]Zhang Y,Liu GQ,Liu XD,et al.Efflux transport of 3HGABA across blood-brain barrier after cerebral ischemia-reperfusion in rats[J].Acta Pharmacol Sin,1999,20(3): 223-226.
[25]Bo Lin,Michael C.Pirrung,Liu Deng,et al.Neuroprotection by Small Molecule Activators of the Nerve Growth Factor Receptor[J].Journal of Pharmacology & Experimental Therapeutics,2007,322(1):59-69.
[26]Capsoni S,Covaceuszach S,Ugolini G,et al.Delivery of NGF to the brain:intranasal versus ocular administration in anti-NGF transgenic mice[J].Journal of Alzheimers Disease Jad,2009,16(2):371-388.
[27] Su Zhengang, Cao Xiaojian, Chen Qi, Fu Zhen. Progress in nerve growth factor crossing the blood-brain barrier [J]. Foreign medical cerebrovascular diseases, 2003,11 (4): 290-293.
[28]Ding Y,Li J,Luan X,et al.Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regiona langiogenesis and cellular overexpression of neurotrophin[J].Neuroscience,2004,124:583-591.
[29] Guan Chenfeng, Lu Wei, Zhang Kaili, Guo Jian, Pan Yifeng, Huang Guodong. Power-loaded growth factor nanoflexible nanolipid-permeable blood-brain barrier [J]. China Medical News, 2014,11 (17): 131-134.
[30] Liu Peng, Wang Jin, Miao Changqing, Liu Yue, Tang Peng, Hou Chen, Zhang Xin, Zhong Li, Li Xiaoqing, Li Rui. Natural changes in neurotrophin expression after focal cerebral ischemia in rats [J]. Stroke and Neurological Diseases, 2015,22 (4): 211-214.
[31]Lindval O,Kokaia Z,Bengzon J,et al.Neurotrophins and brain insults[J].Treade Neurosci,1994,17:490-496.
[32] Zhang Hui, Guan Caixuan, Zhang Chaodong. Expression of NGF and BDNF in the brain of rats with cerebral infarction and diabetes with cerebral infarction [J]. The Journal of Stroke and Neurological Diseases, 2006,23 (2): 210-212.
[33] Wei Menglin, Tian Li, Wang Xiaoqin, Zou Yu'an, Xue Qian. Effects of cerebral ischemia preconditioning on ischemia-reperfusion nerve function in rats [J]. Neuropharmacology, 2016,6 (2): 07-13.
[34] Duan Shurong, Xu Ran, Wang Huihui, Teng Wenli, Wang Desheng, Qi Jiping, Wang Haitao. Expression and significance of TGF- β, VE GF, and NGF after human cerebral infarction [J]. Compilation of the Eleventh National Society of Neurology, 2008,334.
[35] Mai Hui, Yang Zhenjun, Lai Nilin, etc. Effect of murine nerve growth factor on cognitive and motor function in patients with acute cerebrovascular disease [J]. The Journal of Practical Cardiovascular, Brain and Pulmonary Diseases, 2015,23 (4): 29-31.
[36] Fang Qi, Dong Wanli, Xu Zhuan, etc. Clinical observation of exogenous nerve growth factor for the treatment of cerebral infarction [J]. Journal of Soochow University, 2006,26 (6): 958-959.
[37] Zhou Yidong, Wu Yufei. Effect of murine nerve growth factor on neurological and motor function in patients with acute cerebral infarction [J]. Modern practical Medicine, 2015,27 (3): 318-319.
[38] Neurology Branch of Chinese Medical Association, Cerebrovascular Disease Group of Neurology Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of acute ischemic stroke in China 2014 [J]. Chinese Journal of Neurology, 2015,48 (4): 246-257.
[39]Huang RX,Guo YP.Treatment according to the classification and stages of stroke[J].(proposal)Chin J Nerv Ment Dis,2001,27:73-75.
[40]Luigi Aloe,Maria Luisa Rocco,Patrizia Bianchi,Luigi Manni.Nerve growth factor:from the early discoveries to the potential clinical use[J].Journal of Translational Medicine,2012,10:23.
[41]Yuen EC,Mobley WC.Therapeutic potential of neurotrophic factors for neurological disoders[J].Ann Neurol,1996,40:236.
[42] Chen Jie, Li Gengfu, Zi Yanli, etc. Pathophysiological changes of cerebral infarction and the current status of its treatment [J]. Southwest National Defense Medicine, 2011,21 (6): 681-683.
[43] Sun Bin, Liang Haiyan, Chen Ying, etc. Clinical efficacy of murine nerve growth factor therapy in acute cerebral infarction and its effects on serum neuron-specific enolase levels [J]. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine, 2015,25 (9): 822-824.
[44] Zhou Li. Changes of nerve growth factor and its therapeutic outlook after cerebral ischemia [J]. Southwest Military Doctor, 2011,13 (6): 1094-1096.
[45] Zhu Yi, Wang Tong. Biological effects of nerve growth factors and their progress in brain injury rehabilitation [J]. Chinese Journal of Rehabilitation Medicine, 2005,20 (6): 474-476.
[46] Yang Xinzhen. Clinical observation of nerve growth factor [J]. Chinese Journal of Endemic Diseases Prevention and Treatment, 2014,29 (2): 193-194.
[47] Zhang Meng, Wang Chengyan, Chen Jing, et al. Progress in the application of nerve growth factor in ischemic cerebrovascular disease [J]. Journal of Logistics College of the Armed Police Force (Medical edition), 2013,22 (10): 949-952.
[48] Gu Wei, Bi Bao said, Tao Jinzhu, etc. Clinical observation of murine nerve growth factor on brain protection in acute cerebral infarction [J]. Chinese Medical Guide, 2015,17 (4): 356-357.
[49]Hong-Shuo Sun,Michael F Jackson,Loren J Martin,et al.Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia[J].Nature Neuroscience,2009,12(10):1300-1307.
[50] Long Qi, Yin Xiaojuan, Feng Zhichun. Effect of nerve growth factor and basic fibroblast growth factor on endogenous neural stem cells in neonatal rats with hypoxic-ischemic brain injury [J]. Journal of Practical Medicine, 2009,25 (1): 36-39.
[51] Yuan Qionglan, LAN Shunqing, Li Ruixiang, Yang Huijun, Zhang Guangpeng. The expression and significance of TGF- β 1, NGF, and GDNF were up-regulated by activated microglia and macrophages during focal cerebral ischemia in rats [J]. Journal of Stroke and Neurological Diseases, 2001,18 (5): 264-266.
[52] Niu Guangyi, Bai Hongying, Zeng Zhilei, Chen Miaomiao, Liu Huayan. Effect of specific activation of SHH signaling pathway on the expression of NGF and BDNF in rats with acute cerebral ischemia [J]. Journal of Stroke and Neurological Diseases, 2013,30 (10): 895-897.
[53]Akins PT,Liu PK,Hsu CY.Immediate early gene expression in response to cerebral ischemia, friend or foe?[J].Stroke,1996,27(9):1682-1687.
[54]Hattori A,Tanaka E,Murase K,et al.Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells[J].J Biol Chem,1993,268(4):2577-2582.
[55]Takeda A,Onodera H,Sugimoto A,et al.Coordinated expression of messenger RNAs for nerve growth factor,brainderived neurotrophic factor and neurotrophin-3 in the rathippocampus following transient forebrain ischemia[J].Neuroscience,1993,55(1):23-31.
[56]Hsu CY,An G,Liu JS,et al.Expression of immediate early gene and growth factor mRNAs in a focal cerebral ischemia model in the rat[J].Stroke,1993,24(12):178-181.
[57] Huang Jian, Shao Fuyuan, Wu Pingjia, Zhao Zhongxin. Changes in serum nerve growth factor content in acute stroke patients and their clinical significance [J]. Chinese Journal of Elderly Cardiovascular and Cerebrovascular Diseases, 2004,6 (1): 20-20.
